QUICK NAV:
Sacrificial Anode Cathodic Protection
Sacrificial cathodic protection occurs when a metal is coupled to a more reactive (anodic) metal. This connection is referred to as a galvanic couple. In order to effectively transfer corrosion from the metal structure, the anode material must have a large enough natural voltage difference to produce an electrical current flow.
Effective application of cathodic protection can provide complete protection to any exposed areas for the life of the structure. The combination of an external coating and cathodic protection provides the most economical and effective choice for protection of underground and submerged pipelines. For bare or ineffectively coated existing pipeline systems, cathodic protection often becomes the only practical alternative for corrosion protection.
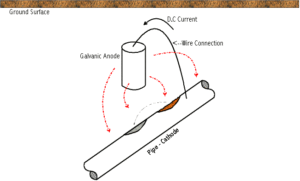
Three metals are commonly utilized for cathodic protection of steel. The selection of the anodic metal is dependent upon resistivity and electrolyte. A general application guide for these metals are:
- Magnesium – soil and freshwater applications
- Zinc – low resistivity soils and saltwater
- Aluminum – saltwater and limited freshwater applications
An advantage of sacrificial anode systems is the flexibility in application. Anodes can be installed in a variety of applications and configurations. No outside power is required for cathodic protection to be effective. Another advantage is the minimal maintenance required for these systems to function.
Disadvantages of sacrificial anode systems include the limited protection current available and limited life. Sacrificial anodes are subject to rapid corrosion (consumption) and require replacement on a regular basis. Typical design life of a pipeline system anode is five to ten years.