QUICK NAV:
Corrosion is usually defined as the deterioration of a metal or its properties caused by a reaction with its environment. Most metals occur naturally in the form of oxides and are usually chemically stable. When exposed to oxygen and other oxidizing agents, the refined metal will try to revert to its natural oxide state. In the case of iron, the oxides will be in the form of ferrous or ferric oxide, commonly known as rust.
Metallic corrosion generally involves the loss of metal at a particular location on an exposed surface. Corrosion occurs in various forms ranging from a generalized attack over the entire surface to a severe concentrated attack. In most cases, it is impossible or economically impractical to completely arrest the corrosion process; however, it is usually possible to control the process to acceptable levels.
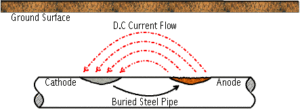
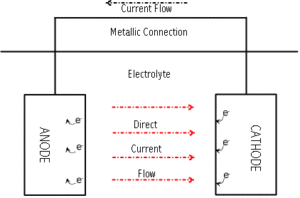
Metallic corrosion is caused by the flow of direct current from one part of the metal surface to another. This flow of direct current causes the loss of metal at the point where current discharges into the environment (oxidation or anodic reaction). Protection occurs at the point where current returns to the metal surface (reduction or cathodic reaction. The rate of corrosion is proportional to the magnitude of the corrosion current. One ampere of direct current removes approximately twenty pounds of steel in one year. Where corrosion occurs and to what extent depends upon the environment to which the metal is exposed.
Four conditions must be met for corrosion to occur. Elimination of any of the four conditions will halt the corrosion reaction.
Corrosion is a natural process. The primary driving force of corrosion is based upon the transformation of iron from its natural state to steel. The refining of iron ore into steel requires the addition of energy. Steel is essentially an unstable state of iron and corrosion is the process of iron returning to its natural state. The energy used in the refining process is the driving force of corrosion.
Corrosion cells are established on underground pipelines for a variety of causes. A primary cause of corrosion is due to an effect known as galvanic corrosion. All metals have different natural electrical potentials. Where two metals with different potentials are connected to each other in a common environment, current will flow causing corrosion to occur. The coupling of steel to a different metal, such as copper, will cause a corrosion circuit to be established. Direct coupling of copper to steel will cause the steel to corrode much faster than normal. Another form of this is the coupling of rusty pipe to new, clean steel. The natural difference in potential causes the new steel to corrode more rapidly than the old steel. Other causes of pipeline corrosion cells include the effect of different soil types, oxygen availability, stray current interference and microbiological growth.
Two other unique causes (and sometimes related) are stress and hydrogen.
Corrosion exhibits itself in a number of ways. A brief description of some of these is provided below.
The five general methods used in the control of corrosion are coating, cathodic protection, material selection, environmental modification, and design practices. Control of underground corrosion is primarily achieved by two methods: coating and cathodic protection. An effective external coating can provide corrosion protection to over 99% of the exposed pipe surface. The protective coating is usually applied to the pipe or tank before burial. The coating serves to electrically insulate the metal from the soil. If the metal could be completely isolated, then the establishment of corrosion cells would be prevented and no corrosion current would flow. However, no coating can be considered a perfect coating. Damage to the coating as a result of handling, transportation, installation, thermal stresses, and soil stresses will eventually create defects or “holidays” that expose the underlying steel to the environment.
Cathodic protection is an electrochemical technique for preventing corrosion of a metal exposed to an electrolyte. The process involves application of DC electrical current to the metal surface from an external source. By forcing the metal surface to accept current from the environment, the underground metal becomes a cathode and protection occurs. The external source can use outside AC power through a rectifier and groundbed or by attaching sacrificial metals such as magnesium or aluminum to the structure to be protected. It is used extensively in preventing corrosion to underground and submerged steel structures; such as pipelines, production well casings, and tanks.
Effective application of cathodic protection can provide complete protection to any exposed areas for the life of the structure. The combination of an external coating and cathodic protection provides the most economical and effective choice for protection of underground and submerged pipelines. For bare or ineffectively coated existing pipeline systems, cathodic protection often becomes the only practical alternative for corrosion protection.
Cathodic protection is a mandated requirement of federal and state regulations governing underground transmission pipelines, gas distribution systems, and underground fuel tanks. These requirements include installation, monitoring, and maintenance of cathodic protection systems.
Cathodic protection is an electrochemical technique for preventing corrosion of a metal exposed to an electrolyte. The process involves application of DC electrical current to the metal surface from an external source. The external source can be either a commercial power source or through connection to sacrificial metals such as magnesium or aluminum. It is used extensively in preventing corrosion to underground and submerged steel structures; such as pipelines, production well casings, and tanks.
Effective application of cathodic protection can provide complete protection to any exposed areas for the life of the structure. The combination of an external coating and cathodic protection provides the most economical and effective choice for protection of underground and submerged pipelines. For bare or ineffectively coated existing pipeline systems, cathodic protection often becomes the only practical alternative for corrosion protection.
Cathodic protection is a mandated requirement of federal and state regulations governing underground transmission pipeline, gas distribution systems, and underground petroleum tanks. These requirements include installation, monitoring, and maintenance of cathodic protection systems.
Cathodic protection essentially means the reduction or elimination of corrosion on a metal surface by forcing the metal to become a cathode. The two general types of cathodic protection systems are impressed current and sacrificial. Both types of systems can effectively transfer the corrosion reaction (oxidation) from the metal surface to an external anode. If all exposed parts of a structure become cathodic with respect to the electrolyte, corrosion of the structure is eliminated.



© 2022 All Rights Reserved MESA