QUICK NAV:
Corrosion Process
Metallic corrosion is caused by the flow of direct current from one part of the metal surface to another. This flow of direct current causes the loss of metal at the point where current discharges into the environment (oxidation or anodic reaction). Protection occurs at the point where current returns to the metal surface (reduction or cathodic reaction. The rate of corrosion is proportional to the magnitude of the corrosion current. One ampere of direct current removes approximately twenty pounds of steel in one year. Where corrosion occurs and to what extent depends upon the environment to which the metal is exposed.
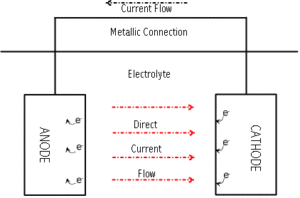
Four conditions must be met for corrosion to occur. Elimination of any of the four conditions will halt the corrosion reaction.
- Anode – the oxidation reaction occurs here. Current discharge into the environment and metal loss are associated with this reaction.
- Cathode – the reduction reaction occurs here. Current acceptance and metal protection are associated with this reaction.
- Electrolyte – the environment to which both the cathode and the anode are exposed. The electrolyte must have the capacity to conduct electrical current through the flow of ions.
- Metallic path – the anode and the cathode must be connected via a metallic connection that conducts electrical current flow through the flow of electrons.